Your Brain Gets Fat Too: The Surprising Role Of Astrocytes In Obesity
- Lidi Garcia
- Jul 9
- 4 min read

Studies in mice show that a diet high in fat and sugar can affect the brain, making eating more impulsive and hindering learning. This happens because of changes in cells called astrocytes, which help regulate behavior and metabolism. Researchers have found that activating these cells can improve memory and blood sugar control, suggesting a new way to treat the effects of obesity on the brain.
Obesity is a very serious and growing public health problem worldwide. It increases the risk of several diseases, such as heart disease, high blood pressure, type 2 diabetes, fatty liver disease, and even some types of cancer.
While genetics and lifestyle (such as a sedentary lifestyle and diet) play a significant role, the extent to which each factor influences can vary greatly from person to person. One of the biggest culprits identified today is the excessive consumption of foods high in fat and sugar, which are very common in modern diets.
Our brain has regions that help control hunger and satiety—that is, when we eat and when we stop eating. But beyond these areas, there's another powerful motivation for eating: the pleasure food provides. This pleasure is linked to the release of dopamine, a chemical associated with feelings of reward, which is also involved in addictive behaviors.
When we eat something delicious, this dopamine is released, activating the so-called "reward system." The problem is that, over time and with the frequent consumption of high-calorie, high-tasting foods, this system can become dysregulated.

Animal studies have shown that, when animals eat this type of food consistently, their eating behaviors cease to be rational and become more impulsive and even compulsive. Furthermore, this diet impairs cognitive flexibility, which is the ability to adapt to changes and think creatively or strategically.
This change is associated with a part of the brain called the striatum, which is important in controlling behavior and emotions. A decrease in dopamine type 2 (D2) receptors has also been observed in this region, which is linked to increased impulsivity.

A cross-section of a mouse brain showing astrocytes (in green) in the striatum. A high-calorie diet and obesity alter the shape of astrocytes, causing them to become reactive, a sign of brain inflammation. © Montalban et al / Nature Communication
Another important finding is the involvement of astrocytes, brain cells that support neurons and participate in the regulation of various functions, in this process. In obesity, brain astrocytes, especially in areas such as the striatum and nucleus accumbens (NAc), show signs of inflammation and functional changes.
These changes occur even before weight gain, indicating that simply consuming high-calorie foods already begins to negatively affect the brain.
In this specific mouse study, researchers analyzed how prolonged exposure to a diet high in fat and sugar (but with the same amount of calories as a regular diet) alters the functioning of astrocytes in the brain.
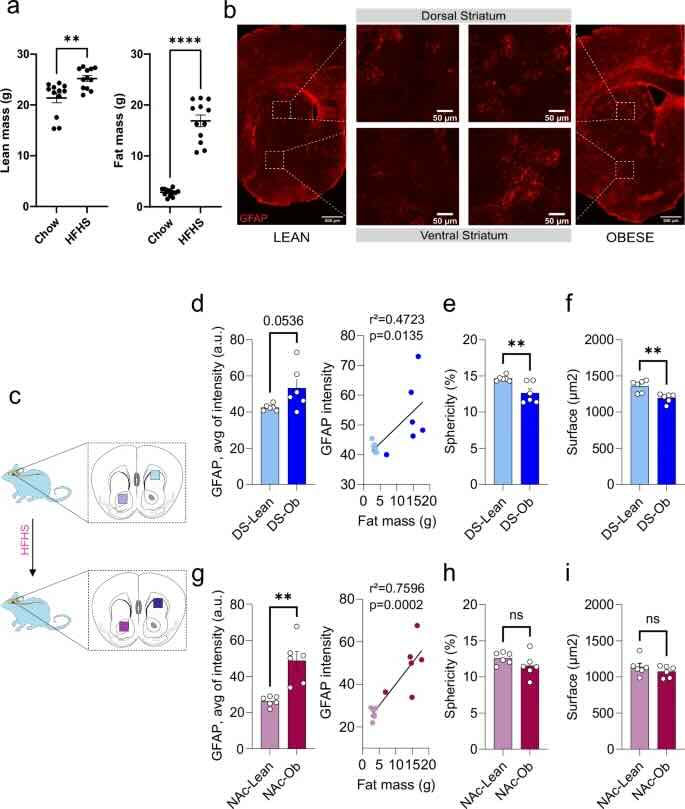
This figure shows that mice fed a high-fat, high-sugar diet (HFHS) for 90 days gained significantly more body fat than mice fed a normal diet. Brain images (in red) show that, in these obese mice, there is an increase in a protein called GFAP, which indicates activation of astrocytes, brain cells involved in support and inflammation, in two specific regions: the dorsal striatum (DS) and the nucleus accumbens (NAc), areas important for behavioral control and reward. Astrocyte activation increased as body fat increased. In the dorsal striatum, this activation was accompanied by changes in the shape of the cells and the area they occupy, suggesting a functional impact. In the nucleus accumbens, although GFAP protein also increased, there was no change in the shape or area of the astrocytes. In summary, diet-induced obesity alters astrocyte activity in specific brain regions linked to motivation and eating behavior.
They found that these changes affect how neurons communicate with each other and impair learning, especially the ability to adapt to new situations (reversal learning).
To test whether these effects could be reversed, the scientists artificially activated (using a technique called chemogenetics) astrocytes in an area of the brain called the dorsal striatum. The results were encouraging: the mice improved their performance on cognitive tests and showed improvements in metabolic functions, such as glucose control.
This shows that these cells play a much more important role than previously thought; they not only influence eating behavior but also help regulate the body's metabolism.

Finally, the study suggests that astrocytes, in addition to being affected by unhealthy diets, may also be key to treating obesity-related problems, such as cognitive impairments and metabolic changes.
This is a promising field of research that could open doors to new treatments in the future, not only focusing on weight loss but also on restoring balance in the brain and body.
READ MORE:
Striatal astrocytes modulate behavioral flexibility and whole-body metabolism in mice
Enrica Montalban, Anthony Ansoult, Daniela Herrera Moro Chao, Cuong Pham, Clara Franco, Andrea Contini, Julien Castel, Rim Hassouna, Marene H. Hardonk, Anna Petitbon, Ewout Foppen, Giuseppe Gangarossa, Pierre Trifilieff, Dongdong Li, Serge Luquet, and Claire Martin
Nature Communications. Volume 16, Article number: 5417 (2025)
Abstract:
Brain circuits in reward-associated behaviors are potent drivers of feeding behavior but also recently emerged as regulator of metabolism. Short or chronic exposures to caloric food alter brain structures and are associated with increased astrocytes reactivity and pro-inflammatory responses in both mice and humans. However, the role of striatal astrocytes in regulating adaptive and maladaptive behavioral and metabolic responses to energy-dense food remains elusive. In this study we reveal that chemogenetic manipulation of the astrocytes in striatal structures can exert a direct effect on peripheral metabolism in male mice, and that manipulation of astrocytes in the dorsal striatum can alter peripheral metabolism and is sufficient to restore cognitive deficit induced by chronic high fat high sucrose (HFHS) diet exposure in obese mice. Altogether, this work reveals a yet unappreciated role for striatal astrocytes as a direct operator of flexible behavior and metabolic control.



Comments