The Gut-Brain Connection: How Probiotics Can Help With Anxiety
- Lidi Garcia
- Feb 24
- 3 min read

Hyperexcitability of neurons in the basolateral amygdala has been linked to a variety of stress-induced anxiety-related behaviors and is associated with gut health. However, the precise causal relationship between gut microbes and anxiety-related behaviors remains unclear. This suggests a potential alternative treatment option to reduce anxiety levels by focusing exclusively on probiotics.
Anxiety is a natural response of the body to stress and is associated with physiological changes that prepare the body for threatening situations. This process is regulated by the hypothalamic-pituitary-adrenal (HPA) axis, which releases stress hormones such as cortisol.
When this system remains activated for long periods, anxiety disorders, characterized by exaggerated reactions to stress, can develop.
Functional magnetic resonance imaging (fMRI) studies have also shown hyperactivity of the basolateral amygdala (BLA) in patients with anxiety disorders.

The basolateral amygdala communicates bidirectionally with brain regions including the prefrontal cortex, hippocampus, nucleus accumbens, and hindbrain regions that influence cognition, motivation, and stress responses.
The hyperexcitability of neurons in the basolateral amygdala has been linked to several stress-induced anxiety-related behaviors.
However, the precise causal relationship between gut microbes and anxiety-related behaviors remains unclear. Previous studies have shown that the gut microbiota, the collection of microorganisms that live in the intestine, can influence behavior and brain chemistry, but it remains unclear how this relationship works.

Researchers at Duke-NUS Medical School in Singapore have studied germ-free (GF) mice, meaning they lack gut microbiota, to understand the impact of these microorganisms on anxiety.
These mice showed elevated levels of anxiety and increased activity in the basolateral amygdala, a brain region essential for processing emotions such as fear and anxiety. This suggests that the absence of microbes may alter neuronal functioning, making the mice more anxious.
In addition, the activity of amygdala neurons was influenced by a specific potassium channel (SK), which is responsible for regulating electrical signals in the brain. In mice without microbiota, this channel was functioning in a reduced manner, leading to greater excitability of amygdala neurons, which contributes to the observed anxious behaviors.
However, when the mice were given live microbes or indole, a metabolite produced by gut bacteria, neuronal activity returned to normal and signs of anxiety decreased. This indicates that certain microbiota-derived compounds may help regulate brain function and reduce anxiety symptoms.
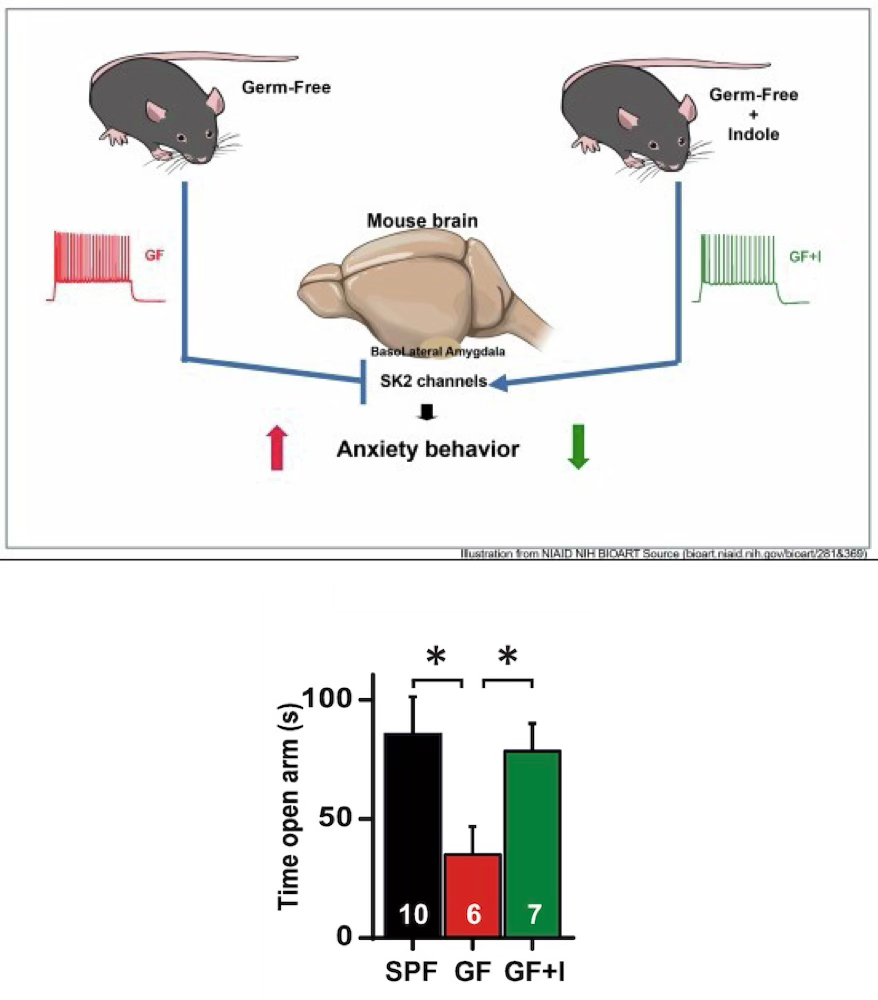
Microbial-derived indoles modulate anxiety and neuronal excitability in the amygdala of male germ-free (GF) mice. These findings demonstrate a molecular mechanism by which microbes regulate anxiety-related behavior. SPF (with microbiota), GF (without microbiota), GF +1 (received microbiota)
The results reinforce the idea that the gut microbiota plays a crucial role in emotional regulation and suggest that therapies based on beneficial bacteria or their metabolites may be a promising approach to treat anxiety disorders.
This discovery paves the way for new therapeutic strategies that can directly target the gut-brain axis, harnessing the potential of the microbiota to balance the stress response.
READ MORE:
Microbial metabolites tune amygdala neuronal hyperexcitability and anxiety-linked behaviors
Weonjin Yu, Yixin Xiao, Anusha Jayaraman, Yi-Chun Yen, Hae Ung Lee, Sven Pettersson and H Shawn Je
EMBO Mol Med (2025) 17: 249 - 264
DOI: 10.1038/s44321-024-00179-y
Abstract:
Changes in gut microbiota composition have been linked to anxiety behavior in rodents. However, the underlying neural circuitry linking microbiota and their metabolites to anxiety behavior remains unknown. Using male C57BL/6J germ-free (GF) mice, not exposed to live microbes, increased anxiety-related behavior was observed correlating with a significant increase in the immediate early c-Fos gene in the basolateral amygdala (BLA). This phenomenon coincided with increased intrinsic excitability and spontaneous synaptic activity of BLA pyramidal neurons associated with reduced small conductance calcium-activated potassium (SK) channel currents. Importantly, colonizing GF mice to live microbes or the microbial-derived metabolite indoles reverted SK channel activities in BLA pyramidal neurons and reduced the anxiety behavioral phenotype. These results are consistent with a molecular mechanism by which microbes and or microbial-derived indoles, regulate functional changes in the BLA neurons. Moreover, this microbe metabolite regulation of anxiety links these results to ancient evolutionarily conserved defense mechanisms associated with anxiety-related behaviors in mammals.



Comments