The Simplest Artificial Cell Ever Created Moves On Its Own
- Lidi Garcia
- Jul 29
- 5 min read

Scientists have created a super-simple artificial cell, made of a bubble with enzymes and a pore, that can move on its own toward certain substances, as if it had a chemical GPS. This discovery shows that even tiny structures can "navigate," helping to understand how early life forms moved and paving the way for technologies like nanorobots that deliver medicines inside the body.
Cells are like tiny living factories: complex, organized, and always functioning. Everything that happens inside us depends on these tiny units. They move, produce energy, reproduce, transport substances… it's as if they have a busy schedule every day.
But what if it were possible to build an artificial cell, something that mimics the basic functions of a real cell, but with as few components as possible?
That's exactly what a group of scientists has achieved: they created an extremely simple synthetic cell that can even "navigate" in response to chemicals in the environment, a behavior known as chemotaxis.
Chemotaxis is essential for many life forms. Think of a bacterium searching for food or a white blood cell traveling to a wound to fight infection. This movement occurs because these cells "sense" chemical signals and move toward them.

Most surprisingly, the researchers showed that even a minimal structure, a simple bubble with a few proteins and enzymes, can do this. This artificial cell has no motor, brain, or flagella, but it still "knows" where to go.
The scientists, led by the Institute of Bioengineering of Catalonia (IBEC), built these artificial cells using lipid vesicles, which are like microscopic bubbles made of fat (similar to the membranes of real cells).

Giuseppe Battaglia (left) and Bárbara Borges (right) at the Institute of Bioengineering of Catalonia (IBEC).
Inside them, they placed enzymes such as glucose oxidase or urease, which are capable of transforming glucose or urea into other substances. To enable these bubbles to interact with their environment, the researchers added a protein called α-hemolysin, which forms a pore in the membrane. This pore acts like a door: it allows molecules to enter and exit.
The genius lies in the asymmetry. Because the enzymes are confined and the pores are in specific locations, chemical reactions do not occur uniformly across the entire surface of the vesicle. This slight imbalance creates a flow around the bubble that pushes it in one direction, precisely toward the highest concentration of the substance it recognizes. It's as if the cell "senses" where there is most food and goes there.
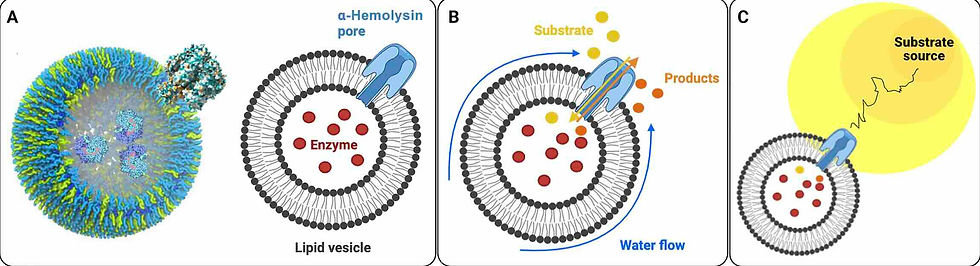
Schematic representation of a minimal chemotactic cell. (A) Lipid vesicles form a compartment used to encapsulate enzymes. The Hly protein, responsible for the toxin, creates pores within the lipid membrane, generating an asymmetry. (B) Substrate molecules react with the enzymes after the enzyme-encapsulating vesicles are immersed in a substrate gradient. The substrate is then converted into product molecules that diffuse preferentially outward through the pore, generating a localized product concentration gradient. This product gradient, in turn, induces fluid flow along the vesicle surface. (C) The fluid sliding velocity on the vesicle membrane sets it in a retreating motion aligned with the direction of the substrate concentration gradient. The relative pore size to the membrane thickness is not to scale.
The team tested this system in the laboratory, within microscopic channels, and tracked the movement of these artificial cells using fluorescence microscopy.
They followed the chemical trail created with glucose or urea, and the effect was even more evident when the vesicles had more pores. In other words, the more "ports" they had, the more sensitive they were to their environment and the better they moved toward what they were "looking for."
This discovery is more than a scientific curiosity. It helps us understand how the planet's first living systems may have moved, long before they developed complex structures like flagella or muscles. It also paves the way for future technologies, such as nanorobots that navigate inside the human body to deliver drugs to precise locations.

Porification of vesicles with Hly. (A and B) Transmission electron microscopy (TEM) images show vesicles (small bubbles that mimic cells) that were incubated with Hly protein. The black arrows indicate Hly proteins that did not bind to the vesicles, while the red arrows show where Hly managed to insert itself into the vesicle membrane, forming small pores. The scale bar represents 50 nanometers (nm), an extremely small measurement. (C) Other TEM images show free Hly protein after incubation with the vesicles. A magnified area shows the pixel intensity profile, that is, the variation in the image along a dashed line that highlights the protein. The red arrows indicate the size of the pore (~4 nm, dashed line) and the outer portion of the protein that resembles a "mushroom cap" (~10 nm, solid line). The scale here is 10 nm. (D) Electron cryomicroscopy images (i.e., with frozen samples) show vesicles with more Hly protein (Hly/PC ratio = 0.5), more clearly indicating the presence of pores. The scale is 50 nm. (E) A test was performed to measure the permeability (i.e., how well the pores function) of vesicles with different amounts of Hly. To do this, they used a fluorescent dye that changes intensity when it interacts with calcium inside the vesicles. The graph shows the fluorescence ratio (F395/F495) measured with ultraviolet light (340 nm) as the amount of Hly increased. In other words, the more Hly, the more substances pass through.
As Professor Lorenzo Battaglia, the research leader, said: "Build simply, understand deeply." By reducing the cell to its bare minimum, a bubble, an enzyme, and a pore, the scientists revealed one of the most fundamental mechanisms of life: how cells know where to go. And this, ultimately, helps us better understand not only who we are, but also where we come from.
READ MORE:
The minimal chemotactic cell
BÁRBARA BORGES-FERNANDES, AZZURRA APRICENO,
ANDRES ARANGO-RESTREPO, SAFA ALMADHI, SUBHADIP GHOSH,
JOE FORTH, JORGE PEDRO LÓPEZ-ALONSO, IBAN UBARRETXENA-BELANDIA, JOSÉ MIGUEL RUBI, LORENA RUIZ-PÉREZ, IAN WILLIAMS, AND GIUSEPPE BATTAGLIA
SCIENCE ADVANCES, 25 Jul 2025, Vol 11, Issue 30
DOI: 10.1126/sciadv.adx9364
Abstract:
The movement of cells and microorganisms in response to chemical gradients, chemotaxis, is fundamental to the evolution of myriad biological processes. In this work, we demonstrate that even the simplest cell-like structures are capable of chemotactic navigation. By encapsulating enzymes within lipid vesicles that incorporate a minimal number of membrane pores, we reveal that a solitary vesicle can actively propel itself toward an enzyme substrate gradient. Specifically, vesicles loaded with either glucose oxidase or urease and embedded with corresponding transmembrane proteins were tracked within a microfluidic device under a controlled substrate gradient. Our findings establish that a system comprising only an encapsulated enzyme and a single transmembrane pore is sufficient to initiate chemotaxis. This proof-of-concept model underscores the minimalistic yet powerful nature of cellular navigation mechanisms, providing a previously unknown perspective on the origins and evolution of chemotactic behavior in biological systems.



Comments