Microglia: The Secret Army That Protects The Brain From Alzheimer's
- Lidi Garcia
- Aug 19
- 3 min read

Scientists have discovered that microglia, the brain's defense cells, need the ADGRG1 receptor to eliminate toxic beta-amyloid plaques linked to Alzheimer's. This receptor activates genes that increase cellular cleansing capacity, protecting neurons and memory. Without it, plaques accumulate rapidly, accelerating the progression of the disease. The discovery could lead to new treatments that strengthen microglia and slow the progression of Alzheimer's.
Alzheimer's disease is a progressive neurodegenerative condition that primarily affects memory, thinking, and behavior. It occurs when abnormal proteins, such as beta-amyloid and tau, accumulate in the brain, forming plaques and tangles that impair communication between neurons and lead to their death.
Over time, this destruction compromises cognitive functions and daily activities, progressing from mild forgetfulness to severe memory loss, disorientation, and motor difficulties. It is the most common form of dementia and has no cure, but research is seeking ways to slow or prevent its progression.

Researchers at the University of California, USA, have discovered a key mechanism that helps the brain defend itself against Alzheimer's disease. The focus is on a special type of immune cell that lives in the brain, called microglia.
These cells act as "janitors" of the nervous system: they identify, engulf, and destroy debris, microorganisms, and abnormal proteins. Among these proteins is beta-amyloid, which can accumulate and form toxic plaques associated with Alzheimer's.
When these plaques are not eliminated, they damage neurons and contribute to memory loss, difficulty thinking, and other symptoms of the disease.

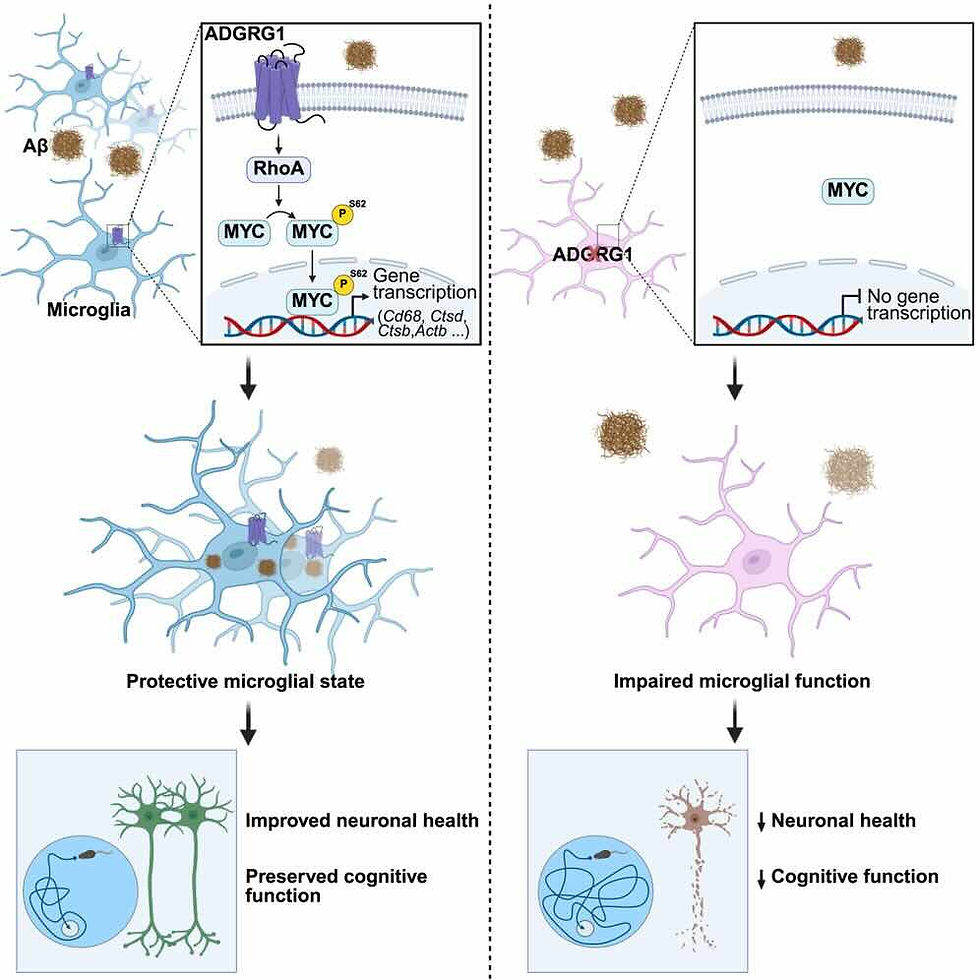
The new research showed that microglia can only perform this function effectively when they have a specific receptor called ADGRG1 on their surface. This receptor acts as a kind of "switch" that activates an internal cellular cleanup program. It activates a transcription factor known as MYC, a protein that regulates which genes are active within the cell.
MYC activation increases the production of proteins linked to phagocytosis (the process of "swallowing" harmful particles) and the activity of lysosomes, which are internal structures responsible for breaking down and recycling captured material.
When the ADGRG1 receptor is present and active, microglia can efficiently eliminate beta-amyloid plaques, reducing the number and size of these deposits and helping to alleviate disease symptoms. However, when the receptor is absent or malfunctions, microglia lose much of this ability.

In experiments with animal models of Alzheimer's disease, the lack of the ADGRG1 receptor led to a rapid increase in plaques, greater neuron loss, and significant impairment in cognitive abilities such as memory and learning.
The scientists also analyzed data from previous studies of human brains. They observed that in people who died with mild-stage Alzheimer's, microglia had high levels of ADGRG1, suggesting the cells were still able to control the disease. In severe cases, however, the levels of this receptor were very low, and the plaques were much more widespread.
The ADGRG1 receptor belongs to a family of receptors called "G-protein-coupled receptors," which are already common targets in drug development for other diseases.
This raises the possibility of creating therapies capable of activating or mimicking the action of this receptor, strengthening microglia so they can fight beta-amyloid more effectively.
In other words, the discovery paves the way for strategies that not only treat symptoms but also help the brain defend itself, keeping its "housekeeping cells" fully functional for longer.
READ MORE:
G-protein-coupled receptor ADGRG1 drives a protective microglial state in Alzheimer's disease through MYC activation
Beika Zhu, Andi Wangzhou, Diankun Yu, Tao Li, Rachael Schmidt, Stacy L. De Florencio, Lauren Chao, Alicia L. Thurber, Minqi Zhou, Zeina Msheik, Yonatan Perez, Lea T. Grinberg, Salvatore Spina, Richard M. Ransohoff, Arnold R. Kriegstein, William W. Seeley, Tomasz Nowakowski, and Xianhua Piao
Neuron. July 25, 2025
DOI: 10.1016/j.neuron.2025.06.020
Abstract:
Germline genetic architecture of Alzheimer’s disease (AD) indicates microglial mechanisms of disease susceptibility and outcomes. However, the mechanisms enabling protective microglial responses remain elusive. Here, we investigate the role of microglial ADGRG1, an adhesion G-protein-coupled receptor (aGPCR) specifically expressed in yolk-sac-derived microglia, in AD pathology using the 5xFAD mouse model. Transcriptomic analyses reveal that ADGRG1 activates the transcription factor MYC, leading to upregulation of genes involved in homeostasis, phagocytosis, and lysosomal functions, thereby promoting a protective microglial state. We demonstrate that deletion of Adgrg1 in microglia impairs MYC activation, resulting in increased amyloid-beta deposition, exacerbated neuronal loss, and cognitive deficits. Functional assays in mouse models and human embryonic stem cell-derived microglia confirm that ADGRG1 is required for Aβ phagocytosis. These findings uncover a GPCR-mediated pathway that drives a protective microglial state via MYC activation, suggesting potential therapeutic strategies to alleviate AD progression by enhancing microglial functional competence.



Comments